How ion exchange resins work
cut lineIon exchange materials are insoluble substances containing loosely held ions which are able to be exchanged with other ions in solutions which come in contact with them. These exchanges take place without any physical alteration to the ion exchange material. Ion exchangers are insoluble acids or bases which have salts which are also insoluble, and this enables them to exchange either positively charged ions (cation exchangers) or negatively charged ones (anion exchangers). Many natural substances such as proteins, cellulose, living cells and soil particles exhibit ion exchange properties which play an important role in the way the function in nature.
Synthetic ion exchange materials based on coal and phenolic resins were first introduced for industrial use during the 1930’s. A few years later resins consisting of polystyrene with sulphonate groups to form cation exchangers or amine groups to form anion exchangers were developed (Figure 1). These two kinds of resin are still the most commonly used resins today.
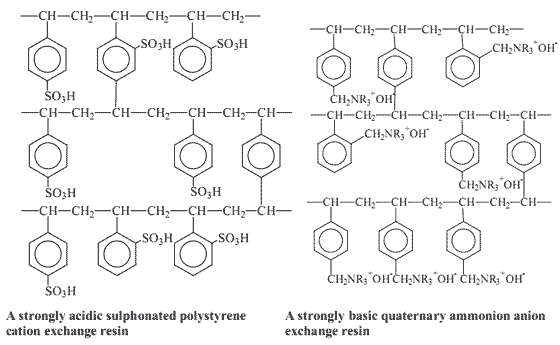
Figure 1 - Some examples of ion exchange resins
The resins are prepared as spherical beads 0.5 to 1.0 mm in diameter. These appear solid even under the microscope, but on a molecular scale the structure is quite open, Figure 2. This means that a solution passed down a resin bed can flow through the crosslinked polymer, bringing it into intimate contact with the exchange sites.
The affinity of sulphonic acid resins for cations varies with the ionic size and charge of the cation. Generally the affinity is greatest for large ions with high valency. For dilute solutions the order of affinity for some common cations is approximately:
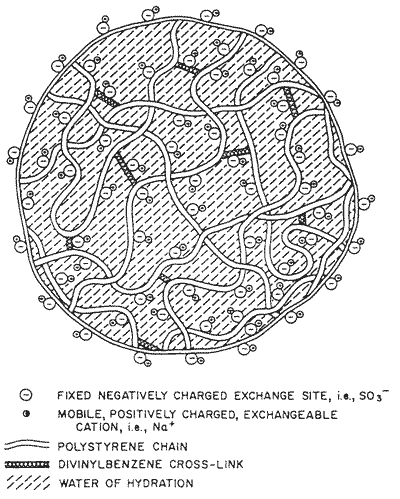
Figure 2 - Expanded view of polystyrene bead
the order of affinity for some common cations is approximately:
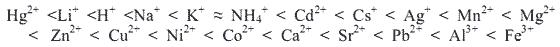
A corresponding list for amine based anion exchangers is:
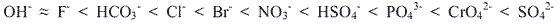
Suppose a resin has greater affinity for ion B than for ion A. If the resin contains ion A and ion B is dissolved in the water passing through it, then the following exchange takes place, the reaction proceeding to the right (R represents the resin):
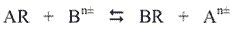
When the resin exchange capacity nears exhaustion, it will mostly be in the BR form. A mass action relationship applies where the bracketed entities represent concentrations:
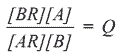
Q is the equilibrium quotient, and is a constant specific for the pair of ions and type of resin. This expression indicates that if a concentrated solution containing ion A is now passed through the exhausted bed, the resin will regenerate into the AR form ready for re-use, whilst ion B will be eluted into the water. All large scale applications for ion exchange resins involved such exhaustion and regeneration cycles.