The uses of ion exchange resin
cut lineA bed of resin can be used either to remove unwanted ions from a solution passed through it or to accumulate a valuable mineral from the water which can later be recovered from the resin. Examples of the removal of unwanted ions are the removal of heavy metals from metal trade wastes, the demineralistion of the whey used to manufacture specialized dairy products and the removal of salts from fruit juices.Strong cation resins in the hydrogen form are used for the hydrolysis of starch and sucrose.Resins also find many uses in the laboratory where the chemistís ingenuity is less constrained by economic considerations. They can be used to remove interfering ions during analysis or to accumulate trace quantities of ions from dilute solutions after which they can be concentrated into a small volume by elution. A cation resin in the hydrogen form can be used to determine the total concentration of ions in a mixture of salts. The sample passing through a column is converted to the equivalent quantity of acid and the amount readily found by titration.
One of the earliest applications of ion exchange was the separation of rare earth elements during the 1940’s. These metals occur naturally as mixtures and have almost identical chemical properties. The equilibrium quotents for cationic resin were found to vary sufficiently for separation to be achieved chromatographically by adding a solution of the mixture to a resin column and eluting the metals with an acid wash. This work lead first to the discovery of promethium (element 61) and later to the discovery of five new elements in the actinide series.
Water treatment
Far more resin is used for water purification than for any other purpose. It is therefore appropriate to discuss water treatment examples when outlining the application of the principles of ion exchange technology. Industrial ion exchange units are produced in sizes ranging from a few litres up to vessels holding several tonnes of resin. Service runs between regenerations usually range from 12 to 48 hours.
The two major types of treatment applied to water are water softening - the replacement of 'hard' ions such as Ca2+ and Mg2+ by Na+ - and demineralisation - the complete removal of dissolved minerals. Both of these treatments are outlined below.
Water softening
In water softening a cation resin in the sodium form is used to remove hard metal ions (calcium and magnesium) from the water along with troublesome traces of iron and manganese, which are also often present. These ions are replaced by an equivalent quantity of sodium, so that the total dissolved solids content of the water remains unchanged as does the pH and anionic content. At regular time intervals the resin is cleaned (Figure 1). This involves passing influent water back up through the resin to remove suspended solids, passing a regenerant solution down through the resin to replace the ions that have bound to the resin and then rinsing again with water to remove the regenerant solution. In water softening the regenerant is a strong solution of sodium chloride.
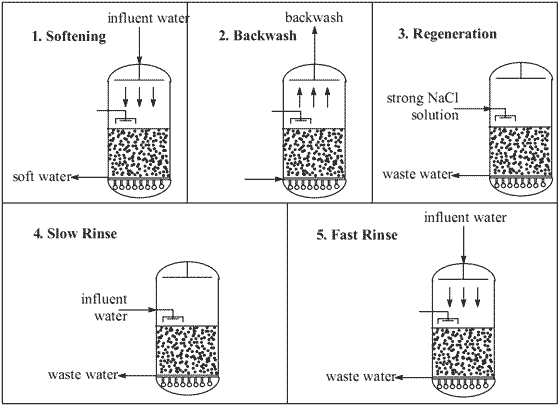
Figure 1 - Using and cleaning a water softening ion exchange system
Demineralization
Virtually all the dissolved matter in natural water supplies is in the form of charged ions. Complete deionization (i.e. demineralisation) can be achieved by using two resins. The water is first passed through a bed of cation exchange resin contained in a vessel similar to that described for softeners. This is in the hydrogen ion form brought about by the use of a strong acid regenerant (either hydrochloric or sulphuric). During service, cations in the water are taken up by the resin while hydrogen ions are released. Thus the effluent consists of a very weak mixture of acids. The water now passes through a second vessel containing anion exchange resin in the hydroxide form for which sodium hydroxide is used as the regenerant. Here the anions are exchanged for hydroxide ions, which react with the hydrogen ions to form water. Such twin bed units will reduce the total solids content to approximately 1-2 mg L-1.
With larger units it is usual to pass water leaving the cation unit through a degassing tower. This removes most of the carbonic acid produced from carbon dioxide and bicarbonate in the feed water and reduces the load on the anion unit. Without degassing the carbonic acid would be taken up by the anion bed after conversion to carbonate.
If complete demineralization is required this is achieved by passing the twin bed effluent through a third vessel containing either cation resin in the hydrogen form or a bed of mixed resin consisting of both anionic and cationic resin which has been intimately combined. Mixed resin is a very efficient demineraliser and can produce water with much lower levels of dissolved material than can be achieved by distillation. For small supplies, such as in laboratories, mixed resin is often used in disposable cartridges. These are only used once, but larger mixed resin units can be regenerated. After exhaustion the bed is subjected to an up flow of water. Anionic resin beads are less dense than the cationic ones and they rise to the top so that the bed is separated into two layers of resin. Each is regenerated in situ with the appropriate regenerant then rinsed with clean water. The internal pipe work of the vessel is arranged so that regenerants and washes enter at the point separating the two resins and flow either up or down as required. An upflow of compressed air then mixes the resins up again.